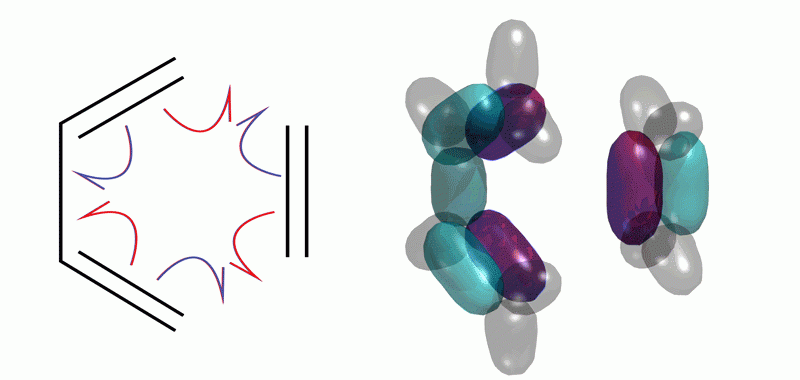
Breakthrough: proving "curly arrows" tell the truth about chemical reactions
Organic chemists can hold their heads up high, knowing that curly arrows now have a basis in "real" theory.
Exciton Science work recently published in Nature Communications bridges the cultural gap between organic chemists and theoreticians that is embodied by the “curly arrow.”
Organic chemists use curly arrows to depict the way chemical reactions occur and the resulting molecular structures. Theoreticians tend to talk about structure of molecules in terms of molecular orbital theory (wave theory) and say that curly arrows are a fiction. The paper unites disparate views of electronic structure for the first time, helping to connect theory with organic chemistry notation.
The team used theoretical modelling, looking at the wave functions in new ways to show why curly arrows work.
“Previously, we knew that curly arrows work but we didn't know why,” research lead Professor Timothy Schmidt of UNSW Sydney says.
According to Data61 collaborator Dr Philip Kilby, who used an optimisation procedure to reduce calculation time by two orders of magnitude, the team was "able to devise a method that can estimate the probabilistic location of electrons as a chemical reaction actually takes place”.
“This allows us to model chemical reactions in a more detailed way than has ever been possible before.”
This unprecedented method of extracting the movements of electrons during a chemical reaction is a breakthrough in connecting traditional depictions of chemical mechanism with state-of-the-art quantum chemical calculations.
“We have shown how to relate rigorous calculations to the qualitative diagrams of organic chemists, in essence showing why they work,” Schmidt explains.
According to UNSW Canberra's Dr Terry Frankcombe, who also collaborated on the project, “this work demonstrates that these classical, intuitive ideas of individual electron movements have been the right way to look at chemical reactivity all along, even though they previously had little support from quantum mechanics”.
Calculating curly arrows from ab initio wavefunctions was published in Nature Communications on Thursday 12 April, 2018.

Curly arrow indicating movement of an electron pair

The Diels-Alder reaction is important in the synthesis of all sorts of pharmaceuticals, including vitamin D. But how does it work? Exciton Science researchers show that it involves the splitting of electron pairs.







